- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
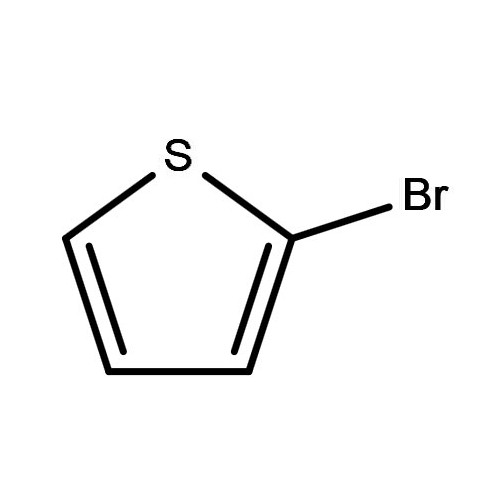
Quare si chemists elige II-bromothiophene pro reactiones?
II, bromothiopheneNumquid a critica heterocyclic compositis late in synthesim de pharmaceuticals, AGROCHEMICAL, et provectus materiae. Et mocecular structuram, featuring a bromine atomus substitutis ad II-situ de Thiophene Annuli, facit illud valde reactive et versatile pro varietate eget mutatio.
Et core utilitatem II-bromothiophene mendacium in facultatem ad participare in crucem-coupling reactiones ut Suzuki, stille et negishi counctions. Haec reactiones sunt essentialis ad construendis complexu moleculis in organicum elit cum princeps praecisione. Dissimilis alias Halogenated Thiophes, II-Bromothiophene stateram reactivity et stabilitatem, ensuring quod potest esse tuto tractari in officinarum et industriae environments sine nimia compositione.
Ex an Industrial perspective, in compositis servit ut aedificium obstructionum in progressionem PROMMIX Polymers et organicum Semiconductors. Hoc facit eam a key component pro innovations in electronic materiae, comprehendo over oversys, photovoltaic cellulis et flexibilia electronic cogitationes. Intelligendo chymicam II-bromothiophene et leveraging suum unicum proprietatibus concedit investigatores ad excogitandum magis agentibus synthety et redigendum unwanted per-products.
Sed et compositis scriptor compatibility cum amplis de solvents, ut DMF, thf, et Toluene, dat flexibilitate in Saccharum strategies. Solubility profile ensures qui profectae procedunt efficiently, providing princeps cedit et reproducibility trans diversis stateram productionem. Hoc facit II-bromothiophenas non solum laboratorium ventus sed etiam certa arbitrium pro magna-scale industriae synthesis.
Quid facit II-bromothiophene munus in organicum synthesim?
Unum ex key quaestiones chemists quaeritur:Quid facit II-bromothiophene augendae reactionem efficientiam et selectivity?Et responsum mendacium in electronic proprietatibus et steric Ordinatio. Et bromine atomi in II-situ crescit electrophilicity anulum, faciens illud magis susceptibilis ad nucleophilic impetum et facilatio formationem C-C aut C-N vinculis in crucem-c vel C-N vinculis in Cross-C-N vinculis in Cross-C vel C-N vinculis in Cross-C-N vinculis in Cross-C, N V vincula in Cross Counter reaction.
Reactionem Applications:
-
Suzuki Counter:Dat formatio Biahl componit cum princeps cedat et selectivity.
-
Stille coitu:Providet iter ad attaching organostannanes ad Thiophene annulum.
-
NFISHI COMPLING:Facilitates reactionem cum organozinc Reagentia pro Advancut Molecule constructione.
In addition, II-bromothiophene scriptor moderata reactivivity concedit chemists ad denique-tune reaction condiciones, optimizing temperatus, catalyst lectio, et solvendo elegit ad minimize parte profectae. Haec praecipue crucial in synthesim de pharmaceuticals ubi puritas et structural integritas sunt paramunt.
Et compositis est etiam favere in heterocyclic Chemiae pro constructione de roued anulus systems. Per opportunum usura II-bromothiophene, chemists potest inducere thiophene unitatum in maius frameworks, quae est de progressionem biologically activae moleculis et eget materiae. Regitur reactividen reducit super-substitutione et permittit precise modificatio in scopum moleculis.
Product parametri:
| Parameter | Specificatio |
|---|---|
| Eget nomen | II, bromothiophene |
| MOLECULA | C4h3brs |
| Pondus | 157.03 G / mol |
| Species | Coloris ad lucem flavo |
| Pudicitia | ≥99% |
| PRAETERITUS | 154-156 ° C |
| Densitas | 1.53 G / CM³ |
| Solubility | Solutum in Organic Solvents (THF, DMF, Toluene) |
Haec parametri II-bromothiophene idoneam utriusque Lab-scale synthesis et magnis, scale industriae applications, cursus constantiam, reproducibility et salus pertractatio.
Quid est II-bromothiophene maluit super aliis halogenated Thiophine?
Eligendo ius Thiophene derivative potest significantly impulsum efficientiam de Saccharum processibus. Ita, quid chemists potius II-bromothiophenas in alternatives sicut III-bromothiophene et II-iodothiophene?
I. Reactivity Libra:
Dum II-Iodothiophene est reactivum, quod etiam magis pretiosa et minus firmum. Bromine praebet idealis statera, offering sufficient reactivity pro crucis-cooptibilibus cum maintaining tractabile tractantem et repono conditionibus.
II. Structural Selectivity:
Substitution ad II-situ directs reactiones in praedictio modo, permittens selectivam functionalization. Hoc praecisione est discrimine ad synthesizing universa moleculis cum princeps cedit et minimal parte products.
III. Cost-efficaciam:
II, bromothiophene est relative parabilis comparari iodinated Analogs. Nam industriae-scale applications, hoc pretium differentia potest significantly impulsum productio budgets sine compromisando qualitas.
IV. Versatility:
Et compositis scriptor compatibility cum variis catalysts, solvents, et reactionem condiciones facit adaptabile ad plures synthetica consilia. Utrum in medicinalibus, materiae scientiae, aut AGROCHEMICUS SYNTHESIR, II-Bromothiophene manet a malle arbitrium.
Commune FAQs de II-Bromothiophene:
-
Q1: Numquid II-bromothiophene tutum est tractare?
A1:Ita cum tractatur secundum vexillum Laboratorium Safety protocols. Staed in refrigerium siccum locum et personalis tutela apparatu commendatur pertractatio. -
Q2: Quid solvents sunt idealis ad reactiones cum II-bromothiophene?
A2:Communis solvents includit tf, DMF, et Toluene, quod offer optimum solubility et suscipio princeps reactionem efficientiam. -
Q3: can II-bromothiophene adhiberi ad magna-scale industriae synthesis?
A3:Absolute. Puritas eius firmitatem et constat efficaciam idoneam utriusque parva scala Laboratory magni scale industriae applications.
Haec FAQs electronica maxime commune de interdum, providing patet et practica ductu pro tutum et effective usus.
Quam ad optimize usum II-bromothiophene in projects
Optimizing II-bromothiophene usus requirit intellectus eius eget proprietatibus, repono requisita, et reactionem mores. Propriis repono in humilis temperaturis et tutela ex humorem ensures in compositis manet firmum in tempore. In Saccharum Applications, lectio ius catalyst et solvente compositum est clavis est maxima reactionem efficientiam.
Nam pharmaceutical synthesis, regere Stoichiometry et reactionem tempore reducit unwanted per-products, ducens ad puriorem finalis products. In materiae scientiae, leveraging eius reactivids in crucem-coupluctions reactiones permittit creaturae de Functionalized Polymers cum amplitate electronic proprietatibus. Inquisitores potest adjust reactionem condiciones ad consequi specifica molecular Architectures, demonstrando compositis scriptor versatility.
Denique Reliability of Suppliers est discrimine elementum in consistent perficientur.LeaciaPuerit summus qualis II-bromothiophenas cum consistent puritatem et comprehensive technica firmamentum. Quorum products sunt tailored in occursum postulationes moderni laboratoriosam et industriae facilities. Ut de tua requisitis vel ponere ordinemContact UsHodie professional ductu et suppleret solutiones.